What is the PICOSI framework?
When conducting evidence synthesis, many individuals use the PICO framework. PICO stands for
population; intervention; comparator(s); and outcome. This helps to describe the research question that the evidence synthesis aims to answer. However, recently Remiro‐Azócar (2024) proposed PICOSI framework. PICOSI stands for:
PopulationInterventionComparator(s)OutcomesSummary effect measure; Intercurrent events
In fact, Canada’s Drug Agency’s (CDA) recent draft methods guide recommends the use of PICOSI when defining a target estimand. While the first 5 components many be familiar, intercurrent events are less well known. CDA defines this as:
…intercurrent events refer to those events of interest that occur after treatment has been initiated that may impact the interpretation of the end point quantifying the treatment effect (e.g., the event modifies the treatment effect, such as in the use of rescue medications or other concomitant treatments, or has implications for adherence to the treatment regimen, including premature treatment discontinuation)
The difference is important because the perspectives of regulators and payers differ.
The HTA paradigm places emphasis on evaluating “real‐world” effectiveness, whereas regulators typically prioritize internal over external validity when assessing clinical efficacy…HTA policy decisions typically consider external validity, but external validity depends on the population that is targeted by the payer
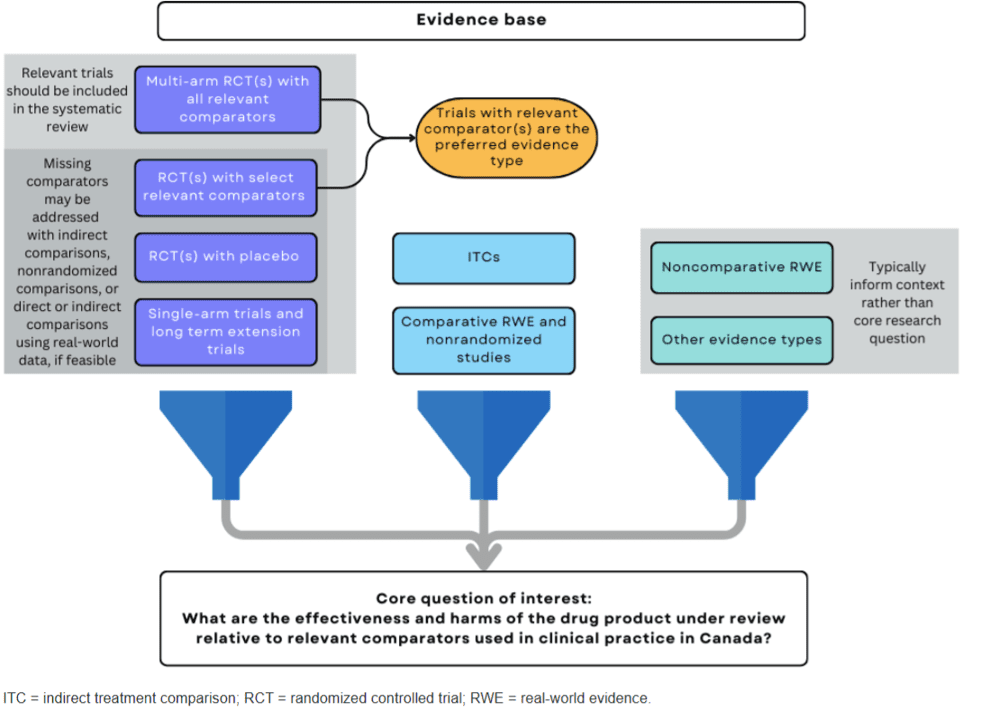
CDA describes the different types of evidence that can be used within an evidence synthesis exercise for HTA purposes in the figure below.
https://www.cda-amc.ca/sites/default/files/MG%20Methods/MG0030-Quantitative-Methods-Manual-Line-Numbered.pdf
Read more about the CDA’s methods guide for technology assessment here.




