Overview of FDA Drugs Approved in 2021

In 2021, the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) approved 50 new drugs, either as new molecular entities (NMEs) under New Drug Applications (NDAs), or as new therapeutic biologics under Biologics License Applications (BLAs). FDA summarizes these approvals in their recently released report titled: Advancing Health Through Innovation: New Drug Therapy Approvals, 2021.
Based on recent trends, 2021 can be seen as an above average year for innovation. Between 2012-2020, the average number of drugs approved was 42.2 (median: 45). Thus, we can consider 2021 as an above average year. This can be thought of as particularly so given potential slow-down in clinical trial due to COVID-19.
https://www.fda.gov/media/155227/download
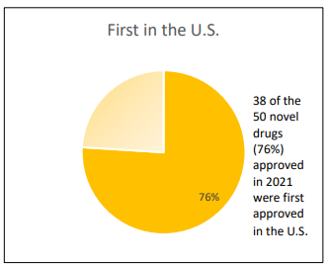
Of interest, 76% (n=38/50) of the novel drugs approved in 2021 were approved in the U.S. before any other country. Part of this is due to the speed of the FDA relative to other regulatory bodies around the world. FDA reports that 86% of approved drugs were approved in their first cycle; FDA claims that this fact “reflects the extent to which CDER staff provide clarity to drug developers on the necessary study design elements and other data needed in the drug application to support a full and comprehensive drug assessment”. However, part of the reason so many new drugs debut in the US is due to pharmaceutical companies strategically deciding to file for approval for in the US, which is the largest pharmaceutical market in the world.
 https://www.fda.gov/media/155227/download
https://www.fda.gov/media/155227/download
We can break down the approvals across a number of other characteristics.
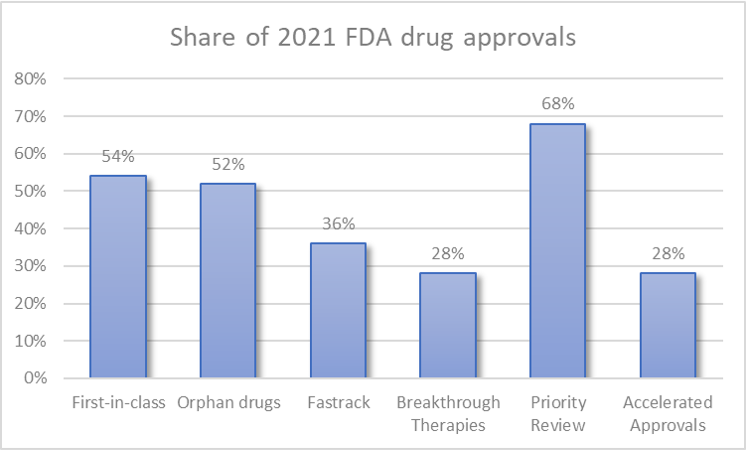
One may be concerned that many of the approvals were “me too” drugs. However, we see that over half (54%, n=27/50) of appovals were for first in class drugs. These approvals included (Adbry, Aduhelm, Besremi, Brexafemme, Bylvay, Cosela, Cytalux, Empaveli, Evkeeza, Kerendia, Korsuva, Leqvio, Livtencity, Lumakras, Lupkynis, Nulibry, Rezurock, Rybrevant, Saphnelo, Tavneos, Tezspire, Tivdak, Verquvo, Voxzogo, Vyvgart, Welireg, Zynlonta). Interestingly, 52% of the approvals (n=26/50) received an orphan drug indication.
More detail on approval designations is below.
Fast track (36%). Fast Track speeds new drug development and review by increasing the level of communication between FDA and drug developers and by enabling CDER to review portions of a drug application on a rolling basis. Drugs included Aduhelm, Amondys 45, Brexafemme, Bylvay, Cabenuva, Cytalux, Empaveli, Exkivity, Kerendia, Lumakras, Lupkynis, Nexviazyme, Rylaze, Saphnelo, Scemblix, Truseltiq, Verquvo, VyvgartBreakthrough Therapy (28%). A Breakthrough Therapy designation includes all the Fast Track program features and offers more intensive FDA guidance on efficiencies for drug development. Relevant drugs included Cosela, Evkeeza, Exkivity, Jemperli, Korsuva, Livmarli, Livtencity, Lumakras, Nexviazyme, Nulibry, Rezurock, Rybrevant, Scemblix, Ukoniq.Priority Review (68%). A drug receives a Priority Review if CDER determines that the drug could potentially provide a significant advance in medical care. This means CDER aims to take action on a drug application within six months of filing (compared to 10 months under standard review).Accelerated approval (28%). The Accelerated Approval pathway provides FDA more flexibility in what endpoints can be used to approve a drug that offers a benefit over current treatments for a serious or life-threatening illness. These accelerated approval endpoints may include those that are “reasonably likely” to predict clinical benefit, which may enable the drug to show benefits over a shorter treatment duration (whereas longer-term demonstration of benefit is needed for traditional approval). Subsequent confirmatory trials must be conducted to support traditional approval. This program aims to bring drugs that can provide important treatment advances sooner to market than with traditional approvals. Drugs approved under this pathway included: Aduhelm, Amondys 45, Exkivity, Jemperli, Lumakras, Pepaxto, Rybrevant, Scemblix, Tepmetko, Tivdak, Truseltiq, Ukoniq, Voxzogo, Zynlonta.
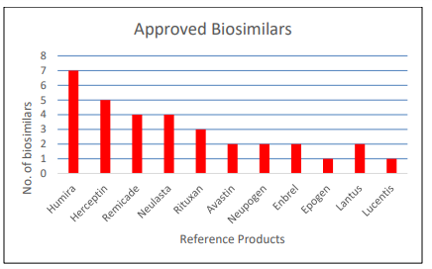
FDA also approved 4 new biosimilar products in 2021. Since 2015, FDA has approved 33 biosimilar products for 11 different reference biologic treatments.
 https://www.fda.gov/media/155227/download
https://www.fda.gov/media/155227/download




