Medicare Drug Price Negotiation leads to 38% to 79% price decrease

Medicare drug price negotiation is here. The maximum fair prices (MFP) set by Medicare lead to discounts between 38% and 79%. In fact, the real discounts are larger since the figures listed in the table below are discounts off of 2023 prices (rather than 2024 prices).
How did the negotiation process work? CMS’s fact sheet described the process as follows:
CMS held three meetings with each participating drug company to discuss the offers and counteroffers, discuss evidence, and attempt to arrive at a mutually acceptable price for the drug. During the course of the negotiation process, CMS revised its offers for each of the drugs upward in response to these discussions. Likewise, many drug companies revised their counteroffers for their drugs downward, based on the discussions with CMS. For five of the selected drugs, this process of exchanging revised offers and counteroffers resulted in CMS and the drug company reaching an agreement on a negotiated price for the drug in association with a negotiation meeting. In four of these cases, CMS accepted a revised counteroffer proposed by the drug company. For the remaining five selected drugs, CMS sent a written final offer to those drug companies, consistent with the process described in its guidance, and in each instance, the drug company accepted CMS’s offer on or before the statutory deadline.
But how exactly did CMS arrive at these prices? This is not clear. In fact, CMS said it won’t make public an explanation of the agreed-upon negotiated prices until March 2025.
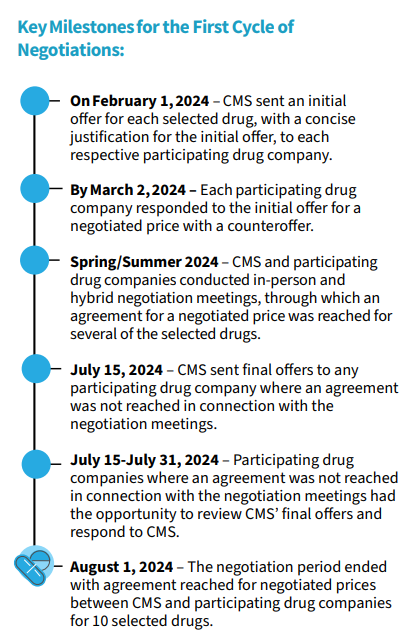
More details on the MFP negotiation timeline is below.
What information and sources were used in setting the MFP? Relevant information included:
…manufacturer=submitted data on research and development costs, prior federal financial support, unit costs of production and distribution, market/revenue/sales data, and information on patents, FDA exclusivities, and FDA applications and approvals. For the factors listed at section 1194(e)(2) related to evidence about alternative treatments (including therapeutic advances, prescribing information, comparative effectiveness, and unmet medical need), CMS considered information from a wide variety of sources, including: information submitted by participating drug companies, people with Medicare, academic experts, clinicians, caregivers, and other interested parties…; information provided at the Patient-Focused Listening Sessions…; information shared by participating drug companies during meetings with CMS; and information CMS identified from its own literature searches, including from clinical guidelines and published studies.
The negotiated prices will be effective January 1, 2026.




