HTA and cell and gene therapy
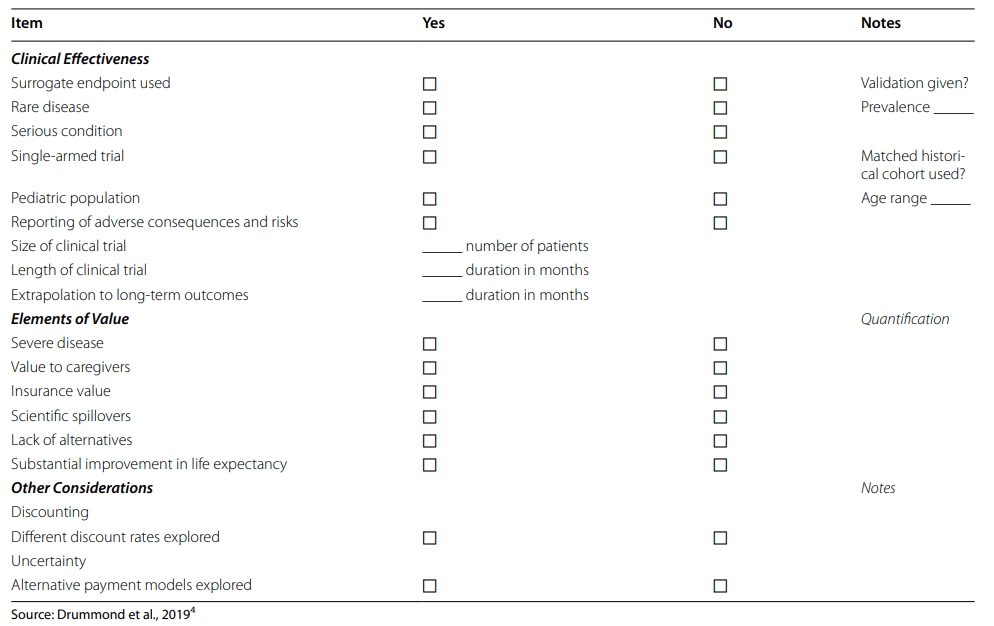
A paper by Drummond et al. (2023) examines how health technology assessment bodies deal with the challenge of value assessment for cell and gene therapies (also known as Advanced Therapy Medicinal Products (ATMPs) in Europe). To do this the authors:
…undertook i) a targeted review of the literature on the clinical and economic evidence needs for these therapies, and ii) an in-depth analysis of HTA reports from 8 major jurisdictions for 9 cell and gene therapies in 10 indications, together with any associated publicly available documents on managed entry agreements and post-launch evidence requirements.
The authors used a data extraction form from Drummond et al. (2019).
The authors examine these elements and determine the share of which these elements were cited in a positive, negative or neutral manner.
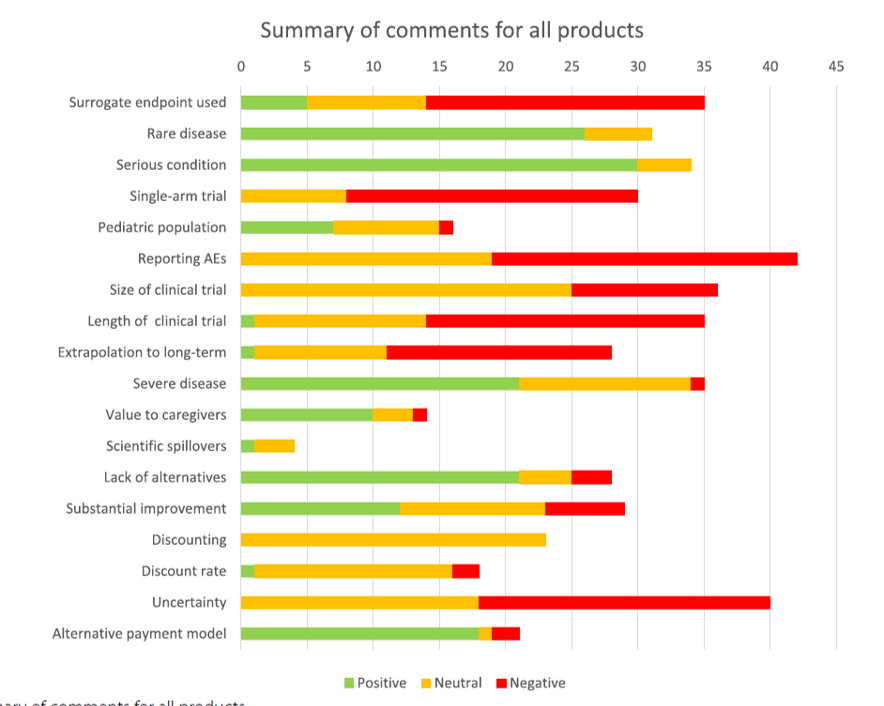
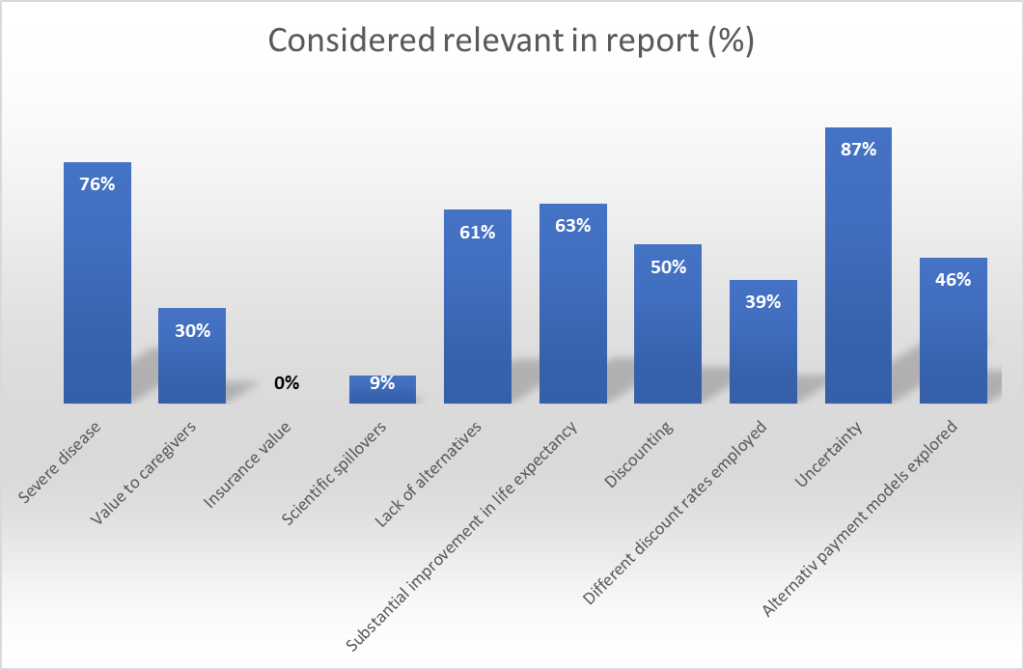
Also of interest is that while some broader or societal value elements were cited in ATMP value assessment, this was far from ubiquitous.
The paper is interesting throughout and you can read the full paper here.




