How many patients are switching to biosimilars?
That is a key question if long-run drug prices are going to come down for biologic products. We can decompose this question into 3 sub-questions:
What share patients initiating therapy start on a biosimilar?What share of patients already using a biologic products switch to a biosimilar?Do patient of physician factors drive biosimilar prescribing patterns?
A paper by Roberts et al. (2024) aims to answer these questions using the medication infliximab as a case study. Infliximab is indicated for a variety of auto-immune diseases (e.g., rheumatoid arthritis, Crohn’s disease, psoriatic arthritis).
Methods
The authors use data from the American College of Rheumatology’s Rheumatology Informatics System for Effectiveness (RISE) registry. RISE is drawn from electronic health records data from >1000 US rheumatologists. The authors run a multilevel logistic regression model clustering patients by practice to examine the share of biosimilar prescribing dependent on patient vs. physician practice factors.
Results
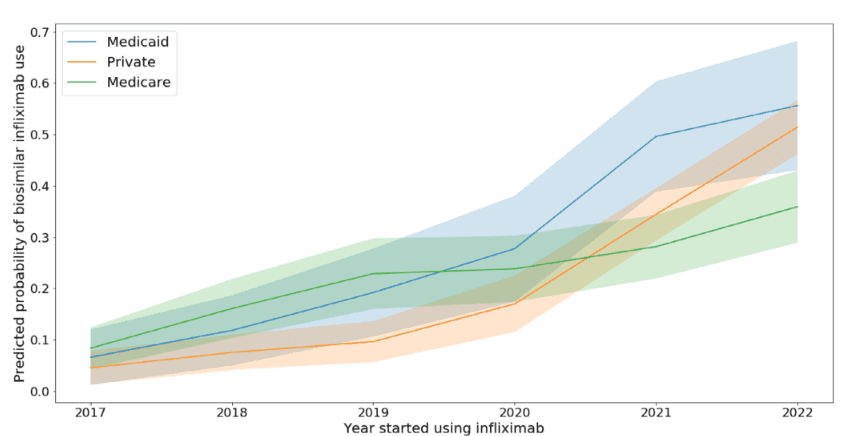
Biosimilar initiation: The authors found that for patients initiating therapy, 21.6% received an infliximab biosimilar between 2017 and 2022. However, while <10% of patients initiated a biosimilar in 2017, by 2022 37% of Medicare patients, 51% of commercially insured and 55% of Medicaid patients initiated a biosimilar version of infliximab. Patients in the lowest socioeconomic status (as measured by Area Deprivation Index) were more likely to initiate a biosimilar (RR = 1.29, 95%CI 1.01–1.66).Biosimilar switching. While there was an increasing trend to more biosimilar treatment initiation, switching from biologic to biosimilar was less common. “86.4% of users who received at least two doses of infliximab stayed with the formulation they were initially prescribed.” Most of the switchers switched from biologic to biosimilar (11.5%) with 1% switching from biosimilar to biologic and <1% switching between biosimilar versions of infliximab. Practice level impacts. The author’s multi-level modelled revealed that 34% of the variation in switching was explained by variation between practices (as measured by the intraclass correlation coefficient [ICC]). “The median new starts on a biosimilar was 16% (IQR: 6%–27%) across practices. Nineteen practices had >40% of new starts on a biosimilar…The median percent of patients switched from bio-originator to biosimilar was 11% (IQR = 5–20%) but 14 practices switched >40% of their patients.”
Predicted probability of starting on biosimilar infliximab among new users of infliximab, by insurance and year of infliximab initiation
https://onlinelibrary.wiley.com/doi/abs/10.1111/1475-6773.14410
Overall, we see a trend towards increased biosimilar prescribing but still significant variability by patient socioeconomic status and insurance type with large variability in biosimilar use across rheumatology practices.




