Health gains from rare disease, specialty and expedited review drugs
James Chambers has put together a series of papers looking at health gains (and cost effectiveness) using the Tufts Cost Effectiveness Analysis Registry. In these studies , he finds that treatments for rare diseases, specialty drugs, and those that receive expedited review are more likely to provide the largest health benefit (≥0.9 QALYs gained versus comparator).
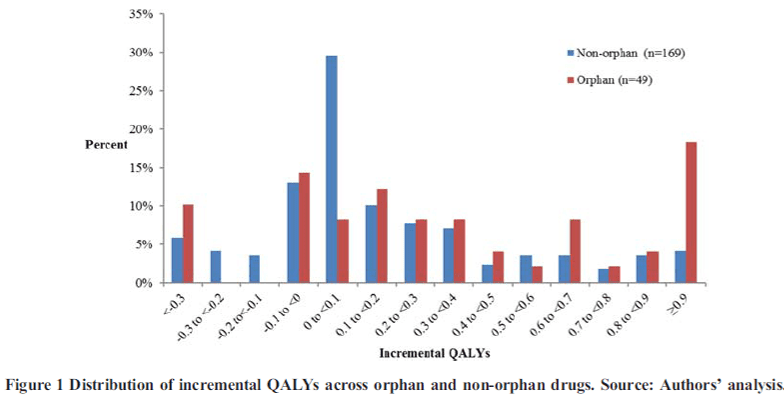
Chambers et al. (2020) shows this is true for treatments for rare disease:
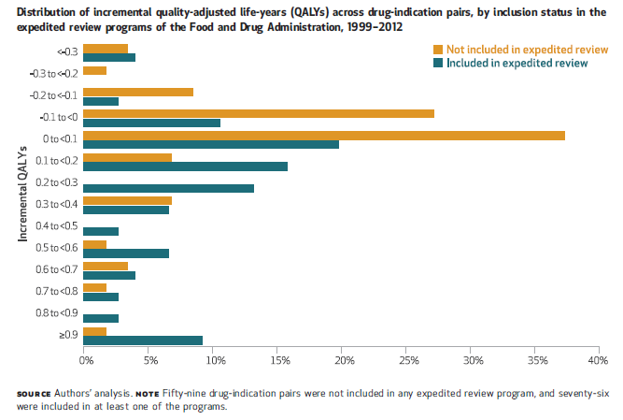
Chambers et al. (2017) shows this is true for treatments receiving expedited review:
and Chambers et al. (2014) shows that this is true for specialty drugs.
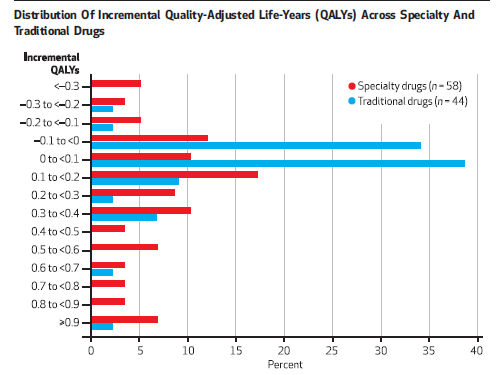
Note that the larger health gains are often correlated with higher drug prices. In the case of rare disease, these highly beneficial treatments are less likely to be cost effective, due to high treatment cost. This should not be surprising, however, as higher prices are needed to fund R&D efforts since–by definition–market sizes are smaller for rare diseases. On the other hand the Chambers et al. (2014) study shows that while specialty drugs cost more, cost effectiveness is comparable to traditional drugs because health gains are higher on average.




