Do first-in-class cancer drugs receive a pricing premium?
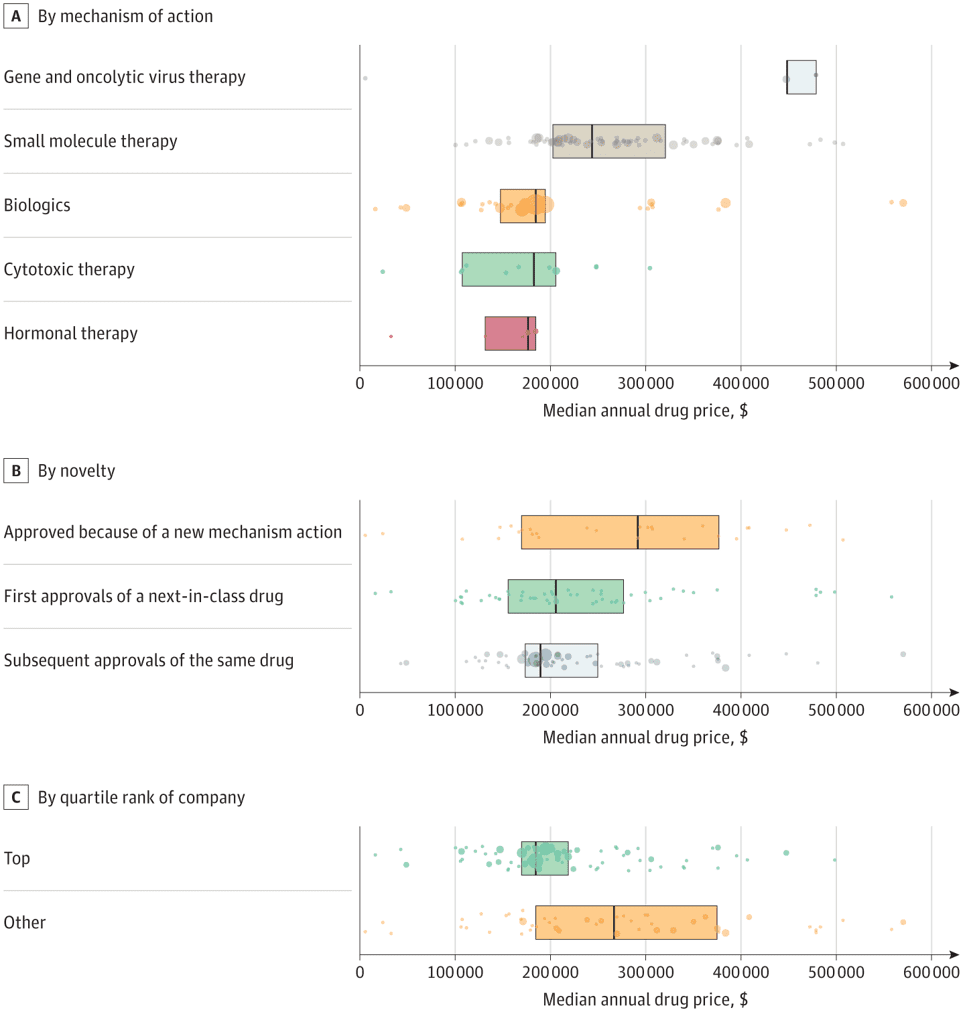
According to a paper by Miljković et al. (2023), the answer is ‘no‘. The authors examine oncology treatments with FDA approvals between 2015 and 2020 and identify average wholesale prices from Redbook. Drugs were classified into three categories: (i) first approval of a new mechanism of action compound, (ii) next-in-class approval regardless the tumor type, and (iii) subsequent approval of the same drug. Using this approach, they found that:
There were 224 cancer drug approvals across 119 individual drugs, with a median annual cost of $196 000 (IQR, $170 000-$277 000). Gene and viral therapies were the most expensive (median, $448 000 [IQR, $448 000-$479 000]), followed by small molecule therapy (median, $244 000 [IQR, $203 000-$321 000), and biologics (median, $185 000 [IQR, $148 000-$195 000]). There was no significant difference in cost between first-in-class, next-in-class, and subsequent approvals of an already approved drug.
The full article is here.




