Considerations in payer coverage of digital therapeutics
The Digital Therapeutics Alliance defines a digital therapeutic (DTx) as “evidence-based therapeutic interventions that are driven by high-quality software programs to prevent, manage, or treat a medical disorder or disease.” One key question is what factors do US payers take into account when evaluating DTx and how does that differ from standard pharmaceuticals. A paper by Gomez Lumbreras et al. (2024) held virtual focus groups with 21 US payers to find the answer. Key considerations include:
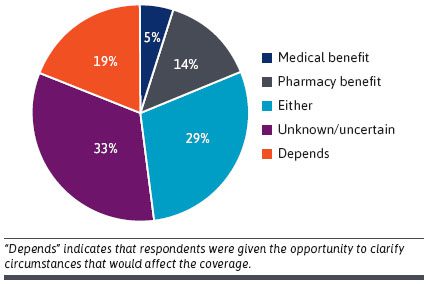
Need for Evidence. Almost all survey respondents (n = 19/21 90%) indicated they would require a clinical trial to consider coverage of the product. This evidence includes data on efficacy, effectiveness and value (including cost-effectiveness perspective)DTx Coverage: Medical, Pharmacy or Other? Many respondents were uncertain if reimbursement should go through medical or pharmacy benefits. The majority thought it would probably be the pharmacy and therapeutics committee (n = 15/21 71%), but , several participants answered “other” (n = 6/21, 29%) [see Figure below]FDA Regulation and Pending Legislation. Overall, 14/21 (66.7%) respondents would require an FDA evaluation of the DTx product for it to be considered for coverage (especially if covered under the pharmacy benefit). Other respondents indicated that FDA evaluation was useful but not always required for coverage. Several payers cited the need for evidence beyond the requirements of the FDA to consider a DTx product for coverage (e.g., effeciveness, value). Reimbursement: NDC vs. CPT. A prescription would be necessary for many health plans to reimburse a DTx product given that many policies exclude reimbursement for over-the-counter products. Participants widely agreed that a coding system would be required, and that a Current Procedural Terminology (CPT) code or National Drug Code (NDC) would be the most efficient ways to ensure reimbursement. Barriers. Barriers mentioned include durability of treatment effect, cost of products, and mechanisms for reimbursement/payment. Other issues included the role of DTx products on patient engagement and treatment adherence. Many perceived that DTx were not “bona fide” treatments in part because some considered them just “apps” and comparable versions could be downloaded online for free. Payer Management. Some claimed that utilization management policies (e..g, prior authorization, step edits, quantity limits) could be used for DTx just as they are for prescription drugs. Others suggested that a DTx product could be part of a care management program rather than covering it separately. A few participants explained that their organizations were currently covering DTx products as part of clinical programs.
Opinions on Under What Benefit Digital Therapeutics Should Be Reimbursed
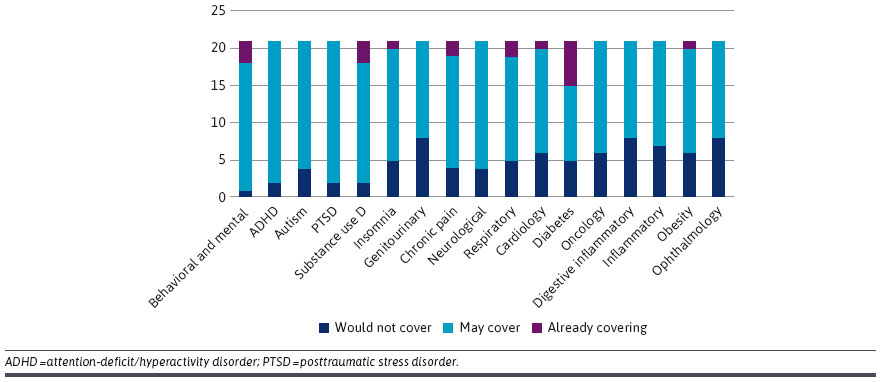 Participants’ Willingness to Cover a Digital Therapeutic Product by Therapeutic Area
Participants’ Willingness to Cover a Digital Therapeutic Product by Therapeutic Area
You can read the full article with helpful quotations here.




