A Strategy For Value-Based Drug Pricing Under The Inflation Reduction Act
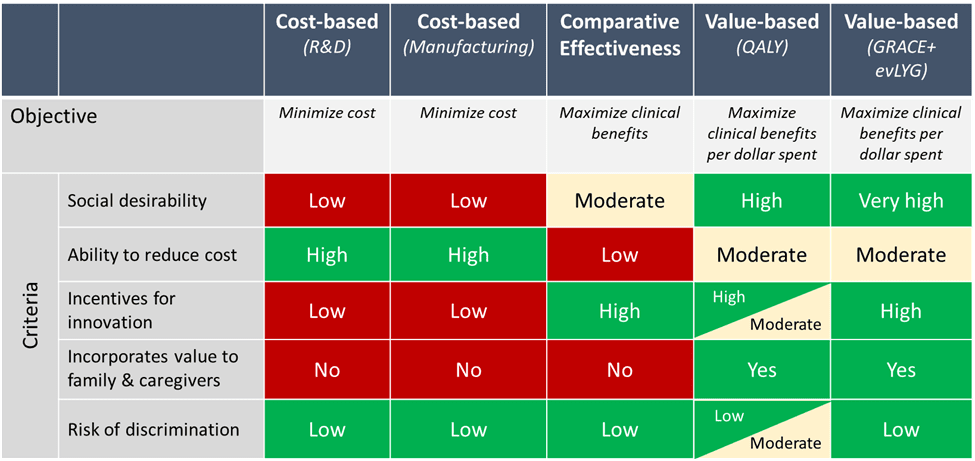
This is the title of my Health Affairs Forefront article published today alongside co-authors Darius Lakdawalla, Jalpa Doshi, Louis Garrison, Anup Malani, Peter Neumann, Charles Phelps, Adrian Towse, and Richard Willke. The article begins by discussing some of the pros and cons of different approaches for CMS to set the maximum fair price (MFP).
Across these options, we recommend using a combination of generalized risk-adjusted cost effectiveness (GRACE) alongside equal value of life years gained (evLYG).
Using GRACE plus evLYG combines the aforementioned three critical factors CMS must consider in a principled manner to comply with the statute’s non-discrimination principle. The only tension between these methods and the CMS memo (p. 50) is that the latter states that “CMS intends to use a qualitative approach to preserve flexibility in negotiation.” We recommend that qualitative analysis be restricted to those considerations that have not been quantified through prior study. Applying a pre-specified quantitative approach to the remaining considerations makes the price negotiation process more transparent and predictable for both patient groups and manufacturers.
More detail describing the rationale for this approach as well a 3-step methodology for implementation are described in the full article here.




