Measuring the novelty of new drugs
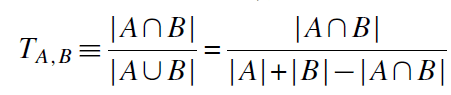
All of us want new, improved medicines. Drugs that provide incremental benefits (often called “me too” drugs) are good, but truly revolutionary therapies are even better. However, how does one separate how “novel” a treatment is? Is there a way to quantify this?
In fact there is. One way to do this is based on the treatment’s molecular structure. For instance, the Tanimoto distance measures the the fraction of chemical features that are shared by the two chemical compounds. Nikolova et al. 2004 summarizes this approach which is calculated as follows:
Krieger, Li and Papanikolau 2022 use this method to quantify how drug development for novel drugs has much more positive informational spillovers on the drug development than incremental drugs. This paper provides an example of an application of Tanimoto distance below.
More det
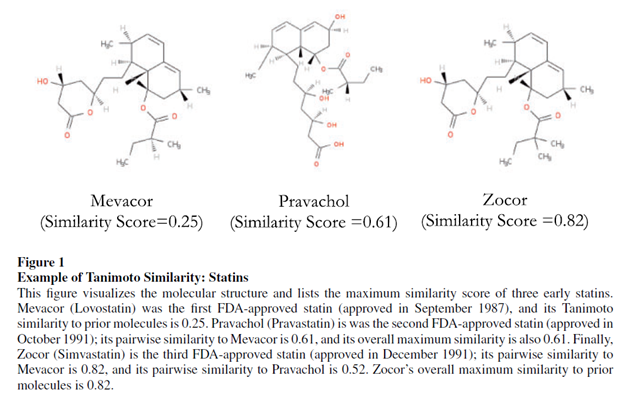
Using the Tanimoto distance as a measure of novelty, the authors find the following:
…novel drug candidates are less likely to obtain FDA approval but are based on more valuable patents. Consistent with a simple model of costly external finance, we show that a positive shock to firms’ net worth leads firms to develop more novel drugs. This suggests that even large firms may behave as though they are risk averse, reducing their willingness to investment in potentially valuable radical innovation.
You can read the full paper here.





