How IRA impact innovation around drugs for orphan disease?
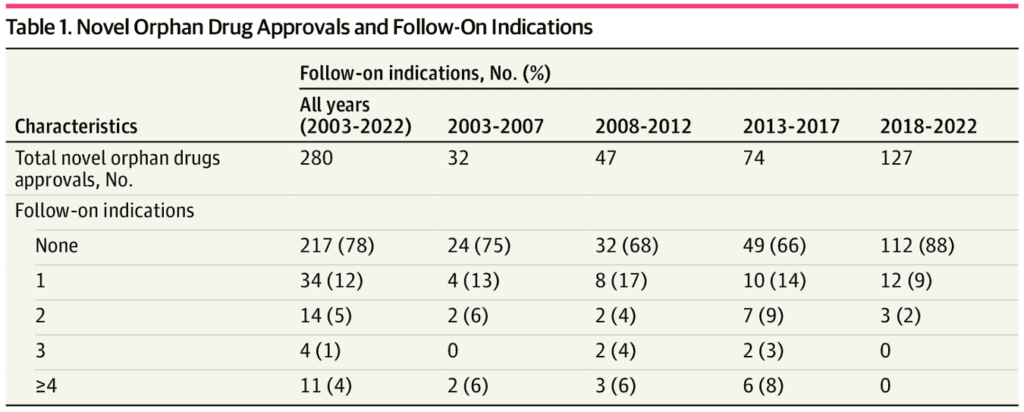
While the Inflation Reduction Act (IRA) aims to lower the prices of pharmaceuticals, at the same time it exempts drugs treating rare diseases (i.e., orphan drugs) from drug price negotiation. However, this exemption is only valid if the orphan drugs has a single approved indication. Thus, a key question is how frequently orphan drugs are later developed for other indications. If so, IRA could could de-incentivize R&D investments in new indications for orphan drugs.
A new paper by Chambers et al. (2023) in JAMA Network Open FDA approval database between 2003 and 2022 to see how common new indications are for orphan drugs. They find that:
FDA approved 282 novel orphan drugs from 2003 to 2022…Overall, the FDA approved 152 separate follow-on indications; 92 (61%) of these follow-on indications were also for orphan drug conditions. The mean (SD) time from novel orphan drug approval to follow-on indication was 53 (43) months…FDA included 58 (38%) follow-on indications in one expedited review program, 46 (30%) in 2 programs, and 17 (11%) in 3 programs; none were included in all 4 programs.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808362
You can read the full article here.





