CMS and outcomes-based pricing for cell and gene therapy
New cell and gene therapies offer the promise of revolutionizing care for patients with genetic diseases. Many of these diseases impact kids and about a third of children in the US are insured by Medicaid or the Children’s Health Insurance Program (CHIP). The government faces a challenge: cell and gene therapies are potential breakthrough therapies but their are costly and the long-term clinical benefits are often uncertain due to (relatively) short duration of clinical trials.
One solution to this issue is outcomes-based contracts. Under outcomes based contracts, payers only pay for cell and gene therapies if they work.
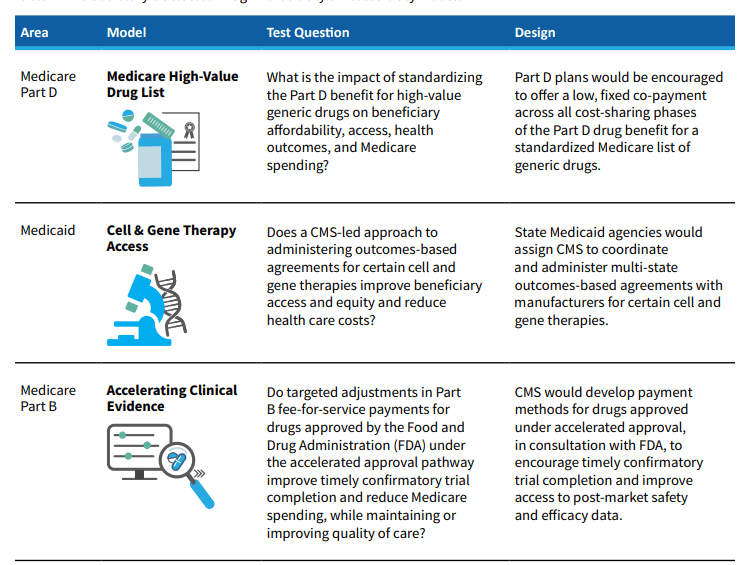
To help State Medicaid Agencies implement this agreement, the CMS’s Innovation Center is considering implementing the Cell and Gene Therapy Access model. CMS describes the program as follows:
The Cell and Gene Therapy Access Model: Cell and Gene Therapies are an emerging area of new drug development that holds significant potential, but these therapies can cost upwards of $1 million. Under this model, state Medicaid agencies would assign CMS to coordinate and administer multi-state, outcomes-based agreements with manufacturers for certain cell and gene therapies. As new therapies come to market, this will help Medicaid beneficiaries gain access to potentially life-changing, high-cost specialty drugs for illnesses like sickle cell disease and cancer.
One benefit for manufacturers of cell and gene therapy is that CMS could coordinate a single outcomes-based contract rather than having to negotiate separately with 50 different State Medicaid Agencies.
A summary of some other Drug Affordability & Accessibility Models are listed below.




