Considerations for coverage of digital therapeutics
What role should digital therapeutics (DTx) play in our treatment landscape? What regulatory and access processes need to be established to be sure these treatments are safe, effective and high value? What evidence is needed to support claims of safety, efficacy and value?
These questions and more were tackled as part of an AMCP Partnership Forum on DTx. The proceedings from this meeting identified a number of areas where DTx oversight and guidance could be improved including:
…standardizing product definitions and categorization across the DTx industry; emphasizing the value of regulatory approval in establishing standards of evidence for DTx; establishing evidence frameworks to guide coverage and reimbursement decisions for DTx; considering unique DTx product aspects such as data security, privacy, and product updates; advancing awareness and professional expertise in DTx among all health care stakeholders; and promoting DTx adoption and equitable access.
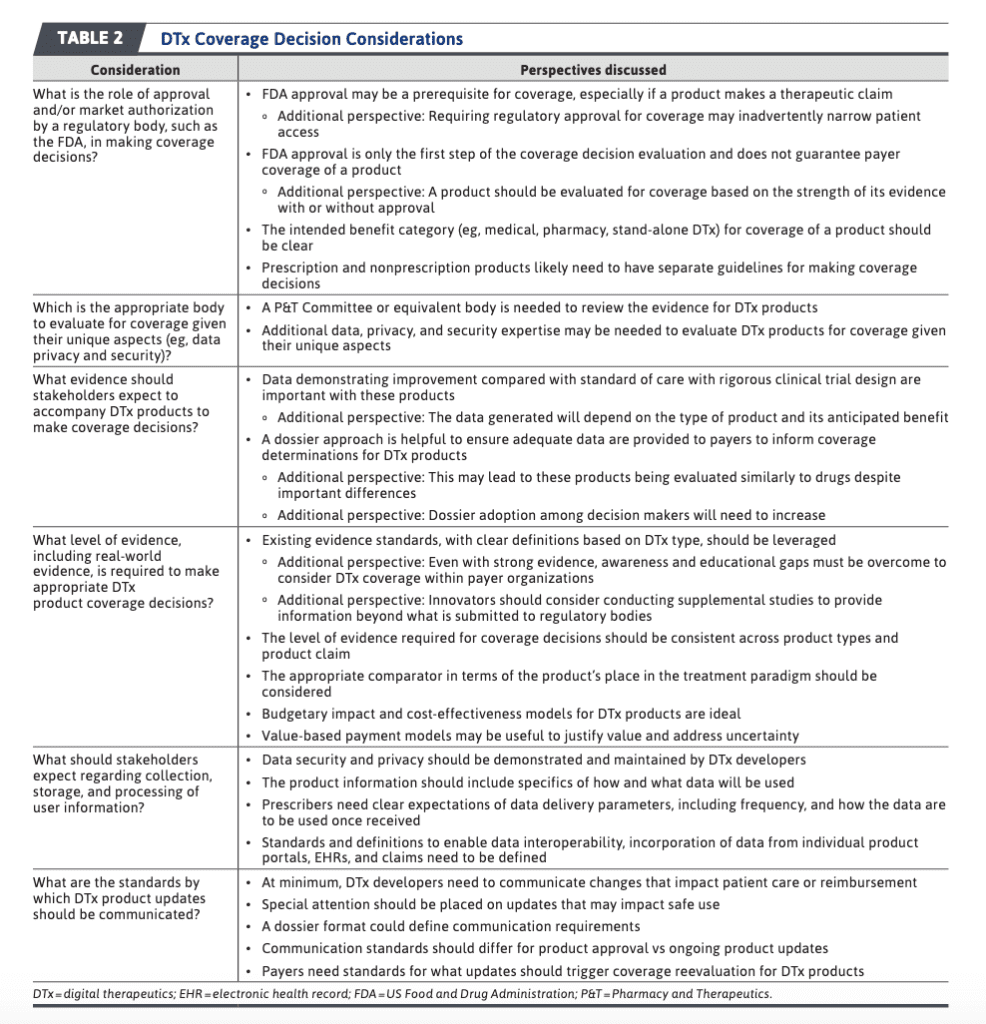
The Abraham et al. (2022) publication also has a nice table, discussing some of the key considerations regulators and payers will face when determining whether to cover a digital therapeutic.
Much more discussion is available at full article.




